When a high power density continuous laser irradiates a tissue, the heating and dessication cause formation of a carbon layer on the tissue surface. The absorption of laser energy by this carbon layer is very efficient and drives tissue vaporization (or "ablation"). This article briefly describes the optical absorption spectrum of such a carbon layer.
A 90-W Nd:YAG laser was used to coagulate some chicken breast from the grocery store (see figure). One site was irradiated until a carbon layer began to form. At a second site irradiation was stopped just prior to the formation of the carbon layer. In both sites, the degree of coagulation was the same.
The optical depth of the carbon layer was measured by a mixed optical fiber bundle carrying light to and from a spectrometer. The optical depth (b, dimensionless) was calculated:
optical depth b = -ln(Rcarb/Rno carb)
The absorption coefficient, μa [cm-1], was calculated:
μa = b/(2d)
where d is the thickness of the carbon layer [cm]. The factor 2 refers to the pathlength for collected photons, one pass through the carbon layer upon entry and a second pass upon escape.
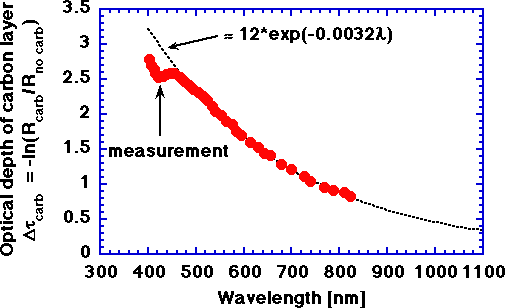
The optical depth of a carbon layer approximately 25 μm thick, a thickness estimate made by Verdaasdonk et al. (University Hospital Utrecht, The Netherlands).
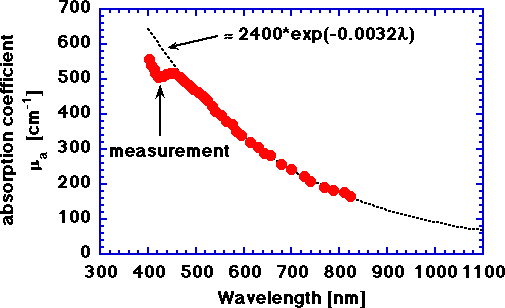
The absorption coefficient of a laser-induced carbon layer on chicken, assuming a 25-μm thick carbon layer. The approximate value of μa for a wavelength, nm, expressed in nanometers is
μa = 2400 exp(-0.0032 nm) [cm-1]
Some values of μa for a few selected wavelengths are:
| Laser Type | Wavelength [nm] | μa [cm-1] |
| argon ion | 488 | 504 |
| argon ion | 514 | 463 |
| HeNe | 633 | 317 |
| diode | 805 | 183 |
| Nd:YAG | 1064 | 80 |
The above values for μa are tentative based on the correctness of the assumption of a 25μm thickness for the carbon layer formed on the chicken. Regardless, the spectral shape as a function of wavelength is correct.