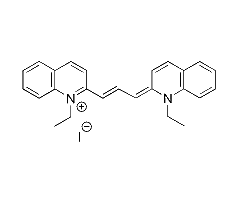
 This page summarizes the optical absorption and emission data of Pinacyanol iodide that
is available in the PhotochemCAD
package, version 2.1a (Du 1998, Dixon 2005). I reworked their data to
produce these interactive graphs and to provide direct links to text files
containing the raw and manipulated data. Although I have tried to be careful, I
may have introduced some errors; the cautious user is advised to compare these
results with the original sources.
This page summarizes the optical absorption and emission data of Pinacyanol iodide that
is available in the PhotochemCAD
package, version 2.1a (Du 1998, Dixon 2005). I reworked their data to
produce these interactive graphs and to provide direct links to text files
containing the raw and manipulated data. Although I have tried to be careful, I
may have introduced some errors; the cautious user is advised to compare these
results with the original sources.
You can resize any of the graphs by clicking and dragging a rectangle. If you hover the mouse over the graph, you will see a pop-up showing the coordinates. One of the icons in the upper right corner will let you export the graph in other formats.
Absorption
This optical absorption measurement of Pinacyanol iodide were made by R.-C. A. Fuh in the summer of 1995 using a Cary 3. The absorption values were collected using a spectral bandwidth of 1.0 nm, a signal averaging time of 0.133 sec, a data interval of 0.25 nm, and a scan rate of 112.5 nm/min.
These measurements were scaled to make the molar extinction coefficient match the value of 128,000cm-1/M at 603.5nm (Rentsch, 1984).
Original Data | Extinction Data
Fluorescence
The fluorescence emission spectrum of Pinacyanol iodide dissolved in methanol. The excitation wavelength was 550nm. The quantum yield of this molecule is 0.001 (Rentsch, 1984). This spectrum was collected by in the summer of 1995 using a Spex FluoroMax. The excitation and emission monochromators were set at 1 mm, giving a spectral bandwidth of 4.25 nm. The data interval was 0.5 nm and the integration time was 2.0 sec.
Samples were prepared in 1cm pathlength quartz cells with absorbance less than 0.1 at the excitation and all emission wavelengths to uniformly illuminate across the sample, and to avoid the inner-filter effect. The dark counts were subtracted and the spectra were corrected for wavelength-dependent instrument sensitivity.
References
Dixon, J. M., M. Taniguchi and J. S. Lindsey (2005), "PhotochemCAD 2. A Refined Program with Accompanying Spectral Databases for Photochemical Calculations, Photochem. Photobiol., 81, 212-213.
Du, H., R.-C. A. Fuh, J. Li, L. A. Corkan and J. S. Lindsey (1998) PhotochemCAD: A computer-aided design and research tool in photochemistry. Photochem. Photobiol. 68, 141-142.
Rentsch, S.; R. Danielius and R. Gadonas (1984) Bestimmung von Lebensdauern und Transientenabsorptionsspektren von Polymethinfarbstoffen aus pikosekunden-spektroskopischen Messungen. J. Signalaufz.-Mater. 12, 319-328.