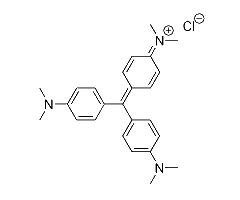
 This page summarizes the optical absorption and emission data of Crystal violet, that
is available in the PhotochemCAD
package, version 2.1a (Du 1998, Dixon 2005). I reworked their data to
produce these interactive graphs and to provide direct links to text files
containing the raw and manipulated data. Although I have tried to be careful, I
may have introduced some errors; the cautious user is advised to compare these
results with the original sources.
This page summarizes the optical absorption and emission data of Crystal violet, that
is available in the PhotochemCAD
package, version 2.1a (Du 1998, Dixon 2005). I reworked their data to
produce these interactive graphs and to provide direct links to text files
containing the raw and manipulated data. Although I have tried to be careful, I
may have introduced some errors; the cautious user is advised to compare these
results with the original sources.
You can resize any of the graphs by clicking and dragging a rectangle. If you hover the mouse over the graph, you will see a pop-up showing the coordinates. One of the icons in the upper right corner will let you export the graph in other formats.
Absorption
This optical absorption measurement of Crystal violet, were made by R.-C. A. Fuh in the summer of 1995 using a Cary 3. The absorption values were collected using a spectral bandwidth of 1.0 nm, a signal averaging time of 0.133 sec, a data interval of 0.25 nm, and a scan rate of 112.5 nm/min.
These measurements were scaled to make the molar extinction coefficient match the value of 112,000cm-1/M at 509.5nm (Zollinger, 1987).
Original Data | Extinction Data
Notes
A fluorescence spectrum was not recorded since most arylmethine dyes have such low emission in fluid solvents. The fluorescence decay depends markedly on solvent viscosity (Cremers, 1980) and the fluorescence yield is 0.019 in the viscous solvent glycerol (Brey, 1977).References
Brey, L. A., G. B. Schuster and H. G. Drickamer (1977) High pressure studies of the effect of viscosity on fluorescence efficiency in crystal violet and auramine O. J. Chem. Phys. 67, 2648-2650.
Cremers, D. A. and M. W. Windsor (1980) A study of the viscosity-dependent electronic relaxation of some triphenylmethane dyes using picosecond flash photolysis. Chem. Phys. Lett. 71, 27-32.
Dixon, J. M., M. Taniguchi and J. S. Lindsey (2005), "PhotochemCAD 2. A Refined Program with Accompanying Spectral Databases for Photochemical Calculations, Photochem. Photobiol., 81, 212-213.
Du, H., R.-C. A. Fuh, J. Li, L. A. Corkan and J. S. Lindsey (1998) PhotochemCAD: A computer-aided design and research tool in photochemistry. Photochem. Photobiol. 68, 141-142.
Zollinger, H. (1987) Color Chemistry: Syntheses, Properties and Applications of Organic Dyes and Pigments, VCH Verlagsgesellschaft mbH, Weinheim, Federal Republic of Germany.