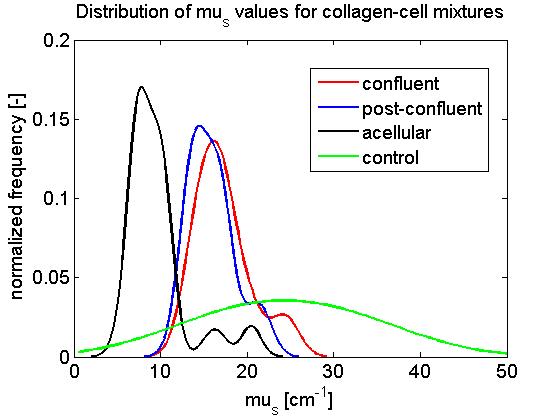
After a week long incubation period, collagen matrices seeded with a cell concentration of 50,000 cells / well grew to confluence (confluent), while collagen seeded with 100,000 and 200,000 cells / well grew beyond confluence (post-confluent). Altogether, each slide was imaged in 5 locations, and each image was subdivided into 3 ROIs laterally spanning 100 μm. The distribution of fitted μs and geff are shown in Figs. 4-5 below.
Fig. 4: Distribution of scattering coefficient μs [mm-1] for various locations on the living optical phantom.
Fig. 5: Distribution of effective anistropy of scatter geff [dimensionless] for various locations on the living optical phantom. Due to the sample arm geometry, the fitting of geff in acellular and control samples (not shown) was not very good, hence these results are very tentative.
Looking at the distribution of fitted μs values (Fig. 4), it can be seen that the scattering coefficient of collagen matrices seeded with cells (confluent and post-confluent) was generally higher than those of unmodified collagen matrices (acellular). It can also be seen that μs values of cell-collagen mixtures in which the cells grew to confluence was not significantly different than those in which cells grew beyond confluence. Control samples which contained collagen and culture medium formed clumpy inhomogeneous suspensions, resulting in a relatively large range of fitted μs values.
In contrast to μs , our extraction algorithm was not as successful at evaluating geff in images of the cell-collagen phantoms (Fig. 5). This can be attributed to a conflict between the sample arm geometry of our OCT system and the light collection scheme necessary in order to extract geff . In our model it can be seen that tissue anisotropy affects the OCT signal in the multiple scattering regime, but not the single scattering regime. Our OCT system has a high numerical aperture lens (a microscope objective) delivering light to the tissue, providing us with improved transverse resolution (~10 μm) but a shorter penetration depth (~400 μm). Thus, we did not collect enough multiple scattered light that would enable accurate extraction of geff . As a result, a large spread of geff values was observed in confluent and post-confluent samples, while it was not possible to evaluate geff from acellular and control samples. Proper fitting of geff would require only a simple redesign of the sample arm.
Overall, our results indicate that cellular remodeling of a collagen matrix affects its optical scattering properties. Moreover, a tentative μs cutoff of 13 mm-1 can be used to distinguish acellular and cellular collagen matrices. Our results suggest that OCT-based optical property extractions can be used to identify regions in vascular construct models that have been remodeled by cells, and that OCT has the potential to become a fast, low-cost, non-destructive optical method of assessing collagen matrix composition in vascular constructs. This method may have important implications in terms of testing vascular constructs prior to implanting them in animals. Further investigations are needed to move OCT towards such a goal.