
© 1998 Steven L. Jacques, Scott A. Prahl
Oregon Graduate Institute

| ECE532 Biomedical Optics © 1998 Steven L. Jacques, Scott A. Prahl Oregon Graduate Institute |
Molecules that absorb light are called chromophores. There are two major types of choromphores:
There are many biological molecules which can absorb light via electronic transitions. Such transitions are relatively energetic and hence are associated with absorption of ultraviolet, visible and near-infrared wavelengths. The molecules generally have a string of double bonds whose pi-orbital electrons act similar to the electrons in a metal in that they collectively behave as a small antenna which can "receive" the electromagnetic wave of a passing photon. If the resonance of the pi-orbital structure matches the photon's wavelength then photon absorption is possible.
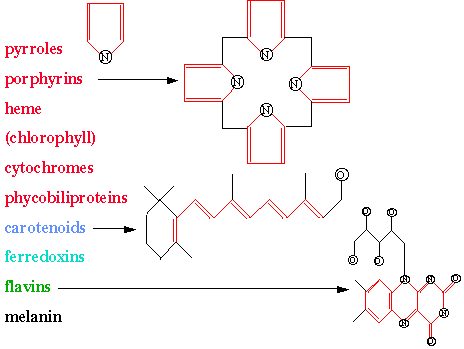
In early biological evolution, the pyrrole molecule was a chromphore which could absorb sunlight which enabled subsequent synthetic reactions that produced biological polymers and other proto-metabolic products. Combining four pyrroles into a tetrapyrrole ring (porphyrin) yielded an efficient chromophore for collecting solar photons. Chlorophyll is such a porphyrin. Hemoglobin, vitamin B12, cytochrome C, and P450 are also examples of porphyrins in biology. The figure lists some common biological chromophores and shows some of their structures. Also see the website spectra which is a compilation of chromophores, most absorbing in the ultraviolet and visible.
The field of infrared spectroscopy studies the variety of bonds which can resonantly vibrate or twist in response to infrared wavelengths and thereby absorb such photons. Perhaps the most dominant chromophore in biology which absorbs via vibrational transitions is water. In the infrared, the absorption of water is the strongest contributor to tissue absorption.
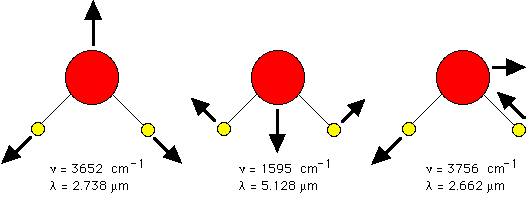
| bond | cycles/cm, 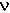 | wavelength, 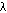 = 1/ = 1/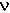
|
| C-H stretch | 2850-2960 [cm-1] | 3.378-3.509 [µm] |
| C-H bend | 1340-1465 | 6.826-7.462 |
| C-C stretch,bend | 700-1250 | 8.000-14.29 |
| C=C stretch | 1620-1680 | 5.952-6.173 |
| C=C stretch | 2100-2260 | 4.425-4.762 |
| CO32- | 1410-1450 | 6.897-7.092 |
| NO3- | 1350-1420 | 7.042-7.407 |
| NO2- | 1230-1250 | 8.000-8.130 |
| SO42- | 1080-1130 | 8.850-9.259 |
| O-H stretch | 3590-3650 | 2.740-2.786 |
| C=O stretch | 1640-1780 | 5.618-6.098 |
| N-H | 3200-3500 | 2.857-3.125 |