
| ECE532 Biomedical Optics
© 1998
Steven L. Jacques, Scott A. Prahl
Oregon Graduate Institute
|
Heat capacity of a cup & stirrer
Let's consider how the combined heat capacity of the cup and stirring bar affects the calibration of the light bulb or laser.
- Assume our cup is a 150-ml glass beaker which has very significant amount of mass (66.58 g) and heat capacity. (A better choice would have been a styrofoam or plastic cup.) Also remember that there is a stirring bar (4.44 g) in the water. So we need to consider the combined mass and heat capacity due to the glass and stirrer.
- Fill the cup with water and let sit at room temperature while being stirred until the water temperature has completely equilibrated with the room temperature. Measure the water temperature as a function of time for a few minutes to ensure stability (Ti = 22.5 °C).
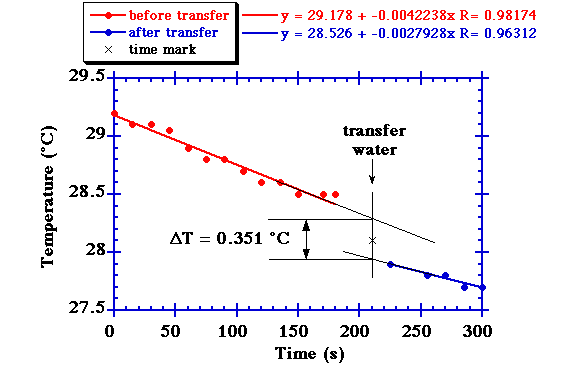
- Fill another cup with exactly 100 ml of warm water. While stirring the cup, measure the water temperature as a function of time for a few minutes. (T1 = 29.2 °C and slowly falling about 0.3 °C per minute).
- Pour out the room temperature water from our room temperature cup and pour all the warm water from the warm cup into the empty room temperature cup. Mark the time of this water transfer.
- Stirring the water, continue measuring the water temperature as a function of time (T2 = 27.9 °C at 15 s after transfer and continues to slowly fall).
- Plot the temperatures T1 and T2 versus time (see figure below). Linearly extrapolate the temperature curves to the time mark of water transfer. At the water transfer, T1 = 28.291 °C and T2 = 27.940 °C. The difference is -0.3515 °C.
- Calculate the difference in energy content, dQ [J], of the 100 ml of warm water versus the energy content of 100 ml of room temperature water:
dQ = rhow*Cw*Vw(T1 - Ti) = (1 g/cm3)(4.18 J/(g °C))(100 cm3)(28.940 - 22.50 °C) = 2461 J
- The above energy is transfered to the empty room temperature cup and heats the cup to a new temperature T2. Calculate lumped factor rhonet*Cnet*Vnet for the combined cup and stirring bar:
rhonet*Cnet*Vnet = dQ/(T2 - Ti) = (2461 J)/(27.940 - 22.50 °C) = 445.0 [J/°C]
- The value of rhonet*Cnet for the 100 ml of water plus glass cup plus stirring bar equals 4.45 J/(g °C), which is 6.5% greater than the value of 4.18 for pure water.
[ Another issue to be considered are evaporative losses from the cup surface. Some experiments were done (not shown here) to demonstrate that the effect of such evaporative losses on this experiment are minor if the temperature rise is only a few degrees.]
Light bulb in black water |
Laser in black water
Power & Energy |
Radiometry


