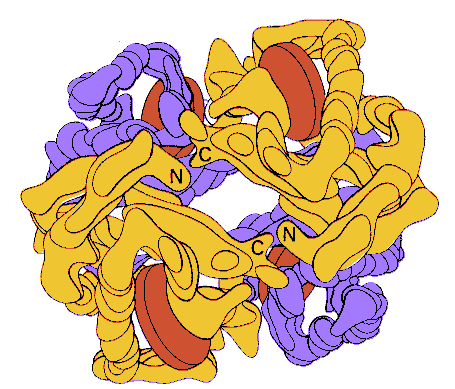
A model of hemoglobin at low resolution. The alpha chains in this model are
yellow, the beta chains are blue, and the heme groups red. From Stryer, Biochemistry who
adapted the picture from M. F. Perutz, The Hemoglobin Molecule (1964).
The Merck Index says
Mammalian hemoglobins have molecular weights of about 64,500. Composed of four peptide chains called globins each of which is bound to a heme. Normal human hemoglobin is composed of a pair of two identical chains. Iron is coordinated to four pyrrole nitrogens of protoporphyrin IX, and to an imidazole nitrogen of a histidine residue from the globin side of the porphyrin. The sixth coordination position is available for binding with oxygen and other small molecules. Called oxyhemoglobin, HbO2 in the oxygenated form and carboxyhemoglobin, HbCO, when the oxygen is displaced by carbon monoxide. Binds reversibly with oxygen while the heme iron remains in the ferrous stae. Autoxidation is prevented by the cover of hydrophobic groups of the globin. When the iron in hemoglobin is oxidized from the ferrous to the ferric state the compound is called methemoglobin and is accompained by loss of oxygen-binding capacity.